 |
Manufacturer |
5.1.1 |
Indicates the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC and EU MDR 2017/745 |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
 |
Date of manufacture. |
5.1.3 |
Indicates the date when the medical device was manufactured |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
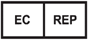 |
Authorized Representative in the European Community / European Union |
5.1.2 |
Indicates the Authorized Representative in the European Community |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
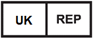 |
Authorized Representative in the United Kingdom |
5.1.2 |
Indicates the Authorized Representative in the United Kingdom |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
 |
Part or Catalog number |
5.1.6 |
Indicates the manufacturer's catalog number so that the medical device can be identified |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
 |
Batch Code |
5.1.5 |
Indicates the manufacturer's batch code so that the batch or lot can be identified |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
 |
Serial number |
5.1.7 |
Indicates the manufacturer's serial number so that the medical device can be identified |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
 |
Medical Device |
5.7.7 |
Indicates the labeled product is a medical device |
MDR 2017/745 – Art. 18 - §1d |
 |
Country of manufacture |
6049 |
Identifies the country of manufacture of products. |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
or
 |
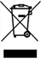
WEEE Waste of Electrical and Electronic Equipment |
N/A |
Indicates that when end user wishes to discard this product it must be sent to separate sent to separate collection facilities for recovery and recycling in the EU |
BS EN 50419:2006 Marking of Electrical Equipment in accordance with Article 11(2) of Directive 2002/96/EC (WEEE) |
 |
United Kingdom Conformity |
N/A |
Equivalent to the EU CE marking. CE marking on a product is a manufacturer's declaration that the product complies with essential requirements of the relevant European health, safety and environmental protection legislation. |
Directive 93/68/EEC CE Marking Directive
EU Medical Device Regulation 2017/745 |
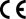 |
CE marking |
N/A |
CE marking on a product is a manufacturer's declaration that the product complies with essential requirements of the relevant European health, safety and environmental protection legislation. |
Directive 93/68/EEC CE Marking Directive
EU Medical Device Regulation 2017/745 |
|
1639
|
CE marking with Notified Body |
N/A |
CE marking on a product is a manufacturer's declaration that the product complies with essential requirements of the relevant European health, safety and environmental protection legislation. |
Directive 93/68/EEC CE Marking Directive
EU Medical Device Regulation 2017/745 |
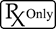 |
US Symbol for prescription only medical devices. |
N/A |
The symbol for Prescription Device
Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician.
|
21 CFR 801.109 |
 |
Consult Instructions for Use |
5.4.3 |
Indicates the need for the user to consult the instructions for use. |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
 |
Consult Electronic Instructions for Use |
5.4.3 |
Indicates the need for the user to consult the electronic instructions for use (eIFU) at the specified website location. |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
 |
Refer to instruction Manual/booklet |
ISO 7010-M002 |
Indicates that the instruction manual / booklet must be read |
IEC/TR 60878 Graphical symbols for electrical equipment in medical practice |
 |
EU Importer |
3725 |
Indicates the entity importing the medical device into the locale |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
 |
Temperature Limit |
5.3.7 |
Indicates the temperature limits to which the medical device can be safely exposed |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
 |
Humidity Limitation |
5.3.8 |
Indicates the range of the humidity to which the medical device can be safely exposed |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
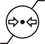 |
Atmospheric Pressure limitation |
5.3.9 |
Indicates the range of atmospheric pressure to which the medical device can be safety exposed |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
 |
Keep Dry |
5.3.4 |
Indicates a medical device that needs to be protected from moisture |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
 |
Caution |
5.4.4 |
Indicates that need for the user to consult the instructions for use for important cautionary information such as warnings and precautions that cannot, for a variety of reasons, be presented on the medical device itself |
ISO 15223-1 Medical devices - Symbols to be used with medical device labels - General Requirements |
 |
Warning: Electricity |
ISO 7010- W012 |
Warns of electricity |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
 |
Equipotentiality |
5021 |
Identifies the terminals which, when connected together, bring the various parts of an equipment or of a system to the same potential, no necessarily being the earth (ground) potential, e.g., for local bonding |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
 |
Current |
5032 |
Indicates on the rating plate that the equipment is suitable for alternating current only; to identify relevant terminals |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
 |
Fuse |
5016 |
Identifies fuse boxes or their locations |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
 |
Standby |
5009 |
Identifies the switch or switch position by means of which part of the equipment is switched on in order to bring it into the stand-by condition |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
 |
ON/OFF (push/push) |
5010 |
Indicates disconnection form the mains, at least for main switches, or their positions, and all those cases where safety is involved. Each position, “ON” or “OFF” is a stable position |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
 |
Type CF defibrillation - proof patient applied part |
5021 |
Identifies a defibrillation-proof CF applied part complying with IEC 60601-1 |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
 |
Battery Pack |
5001B |
Identifies a device related to the power supply by primary or secondary battery, for instance a cover for the battery compartment, or the connector terminals |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
 |
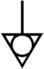
Protective earth, protective ground |
5032 |
Identifies any terminal which is intended for connection to an external conductor for protection against electrical shock in case of fault, or the terminal of a protective earth (ground) electrode |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
 |
Electrostatic sensitive devices |
5134 |
Identifies packages containing electrostatic sensitive devices, or to identify a device or a connector that has not been tested for immunity to electrostatic discharge |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
 |
Type B applied part |
5840 |
Identifies a type B applied part complying with IEC 60601-1 |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |
 |
Non-ionizing electromagnetic radiation |
5140 |
Indicates generally elevated, potentially hazardous, levels of non- ionizing radiation, or to indicate equipment or systems e.g., that intentionally apply RF electromagnetic for diagnosis or treatment |
ISO 7000 / IEC 60417 Graphical symbols for use on equipment |



